Rifampin and HIV: Critical Drug Interaction Insights for Effective Treatment
 Oct, 15 2025
Oct, 15 2025
Rifampin-HIV Dose Adjustment Calculator
Determine Correct Dose Adjustment
Select your current HIV medication to see how rifampin affects it and what dose adjustment is recommended.
Adjustment Recommendation
Imagine a patient battling both tuberculosis (TB) and HIV. One powerful antibiotic kicks TB out, while a cocktail of antiretrovirals keeps the virus in check. What happens when those two treatment worlds collide? Understanding the dance between rifampin and HIV medicines can mean the difference between cure and complications.
Why drug interactions matter in HIV‑TB co‑infection
HIV weakens the immune system, making TB the leading cause of death among people living with HIV. Treating both infections simultaneously is standard, but the medicines don’t operate in isolation. A misstep can lower drug levels, cause toxicity, or spark resistance.
Clinicians need a clear map of which antiretroviral drugs survive the enzyme‑inducing punch of rifampin and which need a dose tweak or a substitute.
How rifampin changes the pharmacology of antiretrovirals
rifampin is a potent inducer of cytochrome P450 enzymes, especially CYP3A4, used as a cornerstone of tuberculosis therapy. When a patient takes rifampin, the liver speeds up the metabolism of many drugs that rely on CYP3A4, shaving off their blood concentrations.
Most modern antiretroviral therapy (antiretroviral therapy is the combination of drugs that suppress HIV replication and preserve immune function) includes agents metabolized by CYP3A4. The result? Sub‑therapeutic levels, viral rebound, and a higher chance of resistance.
The enzyme responsible for the interaction, CYP3A4 is a major liver enzyme that metabolizes over half of all prescription drugs, can be boosted up to three‑fold by rifampin. That boost forces clinicians to either increase the antiretroviral dose or switch to a drug less affected by CYP3A4.
Impact on different ART classes
Not all HIV drugs react the same way. Below is a quick reference that shows the most common classes, a representative drug, how rifampin affects it, and the recommended clinical action.
| ART class | Representative drug | Effect of rifampin | Recommended adjustment |
|---|---|---|---|
| Protease inhibitor (PI) | Lopinavir/ritonavir | Plasma levels drop 70‑90% | Switch to ritonavir‑boosted darunavir or double the dose of lopinavir (with careful monitoring) |
| Non‑nucleoside reverse transcriptase inhibitor (NNRTI) | Efavirenz | Levels reduced ~30% | Increase efavirenz to 800mg daily (only if tolerable) |
| Integrase strand transfer inhibitor (INSTI) | Dolutegravir | Reduced exposure by ~50% | Raise dolutegravir to 50mg twice daily |
| Nucleoside reverse transcriptase inhibitor (NRTI) | Tenofovir disoproxil fumarate | Little to no effect | No change needed |
These adjustments are drawn from the latest WHO is the World Health Organization, which issues global treatment guidelines for TB and HIV and CDC recommendations as of 2024.
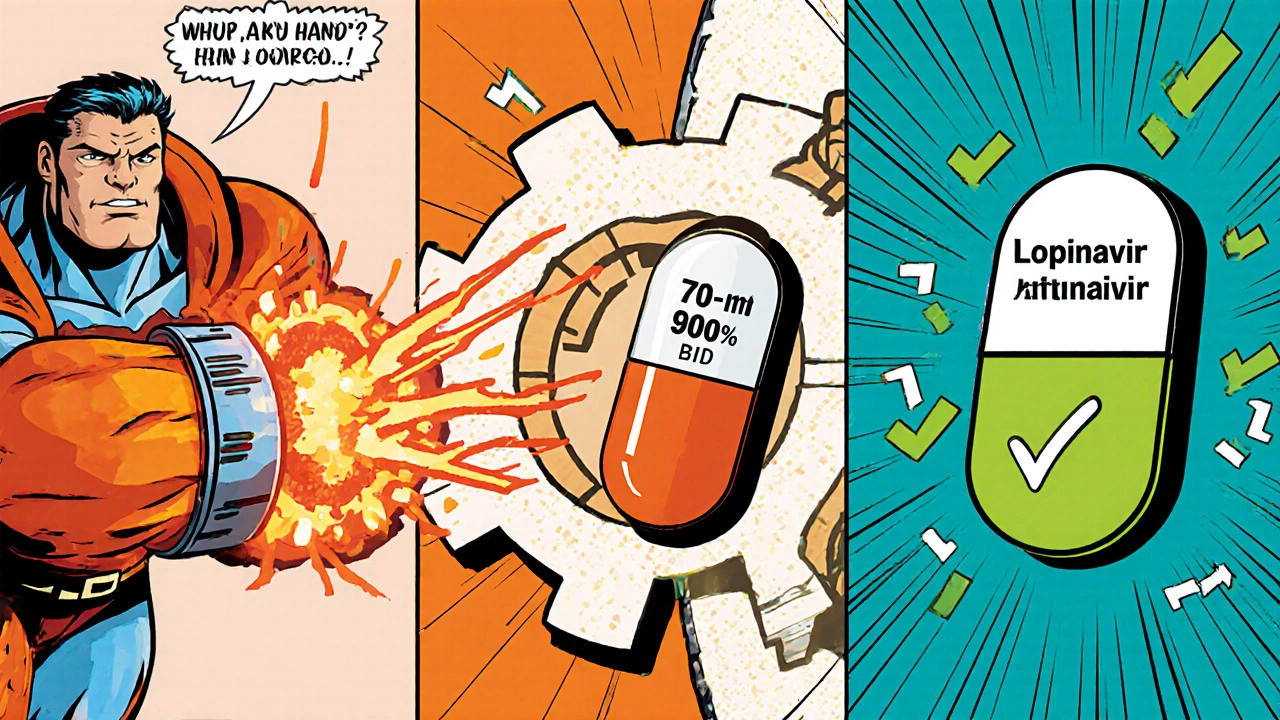
Clinical guidance for dosing adjustments
When you spot a rifampin-ART clash, follow a step‑wise approach:
- Identify the ART regimen the patient is already on.
- Check if the key drug is a CYP3A4 substrate (most PIs, some NNRTIs, and INSTIs).
- Decide whether to up‑dose, switch, or replace rifampin with a less‑inducing alternative like rifabutin.
- Document the new dose and set a follow‑up visit within 2‑4 weeks.
- Order therapeutic drug monitoring (TDM) if available, especially for boosted PIs.
For example, a patient on dolutegravir 50mg once daily should be shifted to 50mg twice daily while on rifampin. If tolerance becomes an issue, consider switching to a rilpivirine‑based regimen, which is less impacted.
Monitoring and managing side effects
Increasing doses can raise the risk of side effects. Keep an eye on:
- Gastro‑intestinal upset (common with higher PI doses).
- Liver enzyme elevations - rifampin alone can raise ALT/AST, and boosted regimens add to that load.
- Neurological symptoms - high‑dose efavirenz may cause vivid dreams or dizziness.
- Renal function - although NRTIs aren’t affected by rifampin, they still need periodic creatinine checks.
If toxicity spikes, re‑evaluate the regimen. Sometimes swapping rifampin for rifabutin (which is a weaker inducer) solves the problem while still treating TB effectively.
Alternative strategies when interactions become too risky
Rifabutin, at a dose of 300mg three times weekly, offers similar TB efficacy with a milder enzyme‑inducing profile. It’s a good pick when a patient is on a PI‑based regimen that can’t be safely up‑dosed.
Another route is to delay ART initiation until the intensive phase of TB therapy is complete (usually 2 months). This is rarely preferred now, as early ART improves survival, but it remains an option for severe drug‑interaction scenarios.
Patient counseling tips
Patients often wonder why their pill count changes. Explain in plain language:
- “Rifampin speeds up how fast your HIV medicine leaves your body, so we need to give a little more to keep the virus suppressed.”
- Emphasize adherence - missed doses of the boosted regimen can quickly lead to resistance.
- Warn about possible side effects from higher doses and tell them when to call the clinic.
Providing a written schedule and a simple chart (like the table above) can boost confidence.
Frequently Asked Questions
Does rifampin affect all HIV drugs the same way?
No. Rifampin mainly lowers the levels of drugs metabolized by the CYP3A4 enzyme. Protease inhibitors, some NNRTIs, and integrase inhibitors are hit hardest, while nucleoside reverse transcriptase inhibitors stay pretty steady.
Can I just double the dose of my HIV meds when I take rifampin?
Doubling works for some drugs (like dolutegravir) but not for others. For protease inhibitors, simply doubling can cause toxicity without fixing the interaction. Always follow guideline‑based adjustments or switch to an alternative regimen.
Is rifabutin safer to use with HIV medicines?
Rifabutin is a weaker inducer of CYP3A4, so it interferes far less with most antiretrovirals. It’s often the go‑to substitute when a patient can’t tolerate dose changes or when drug‑level monitoring isn’t available.
How often should I get blood tests while on rifampin and ART?
Check liver enzymes at baseline, then at weeks 2 and 4 after starting rifampin. If you’ve increased an ART dose, add a therapeutic drug level check (if available) and repeat viral load testing at 4‑6 weeks.
What should I do if I experience side effects after the dose change?
Contact your provider right away. They may lower the dose, add a supportive medication, or consider swapping rifampin for rifabutin. Never stop either drug on your own.
Dervla Rooney
October 15, 2025 AT 13:06I appreciate the concise overview of rifampin’s impact on ART.
Johnny Ha
October 17, 2025 AT 06:46Look, the pharma giants don’t want you to know that rifampin can screw up your HIV meds on purpose. They’re pushing the cheap combo so they can keep their profits high, no matter the side‑effects. The guidelines are just a front to keep us taking more pills at higher doses. If you ask the right people you’ll see how the whole drug‑interaction story is a controlled narrative. Bottom line: stay skeptical and demand alternatives.
Mary Cautionary
October 19, 2025 AT 00:26The pharmacokinetic interplay delineated herein exemplifies a quintessential paradigm of enzymatic induction. By virtue of rifampin’s potent CYP3A4 activation, the systemic exposure to protease inhibitors is markedly attenuated. Consequently, therapeutic regimens necessitate judicious modification to forestall virologic failure.
Crystal Newgen
October 20, 2025 AT 18:06That explanation captures the core issue without overcomplicating matters.
It’s helpful to keep the focus on patient adherence.
Hannah Dawson
October 22, 2025 AT 11:46Analyzing the data reveals a glaring oversight: many clinicians overlook the quantitative impact of rifampin’s induction on dolutegravir’s AUC. The omission is not benign; sub‑therapeutic levels translate to measurable increases in viral load within weeks. Moreover, the risk stratification for hepatic toxicity is understated in the current guidelines. One must interrogate why these gaps persist across diverse healthcare settings.
Julie Gray
October 24, 2025 AT 05:26Undoubtedly, the omission is orchestrated by entities vested in maintaining a monopoly over antiretroviral formulations. The selective dissemination of dosing adjustments aligns with a broader agenda to limit generic accessibility. By restricting transparent data, these forces manipulate clinical practice to favor patented products. It is incumbent upon the informed practitioner to seek independent verification beyond the sanctioned literature.
Lisa Emilie Ness
October 25, 2025 AT 23:06Great summary of the interaction
Emily Wagner
October 27, 2025 AT 15:46The dance between rifampin and antiretrovirals is akin to a dialectic of metabolism, where the enzyme acts as a thesis and the drug as an antithesis.
When the catalyst asserts its influence, the resultant synthesis yields a new equilibrium, though not always a beneficial one.
Understanding this interplay requires both empirical data and a philosophical openness to complexity.
Thus, clinicians become the mediators of this biochemical discourse.
Mark French
October 29, 2025 AT 09:26While the article presents a thorough overview, its practical implications warrant further clarification.
Clinicians must balance the rigour of dosing adjustments with the realities of patient adherence, especially in resource‑limited settings.
Its importance cannot be overstated, and i hope future revisions will address these nuances.
Daylon Knight
October 31, 2025 AT 03:06Oh sure, because everyone loves double‑dosing their meds just for fun
Jason Layne
November 1, 2025 AT 20:46Rifampin’s role as a CYP3A4 inducer is well‑documented, yet the mainstream narrative trivializes its clinical ramifications.
First, the reduction in protease inhibitor concentrations can precipitate virologic rebound within a matter of weeks.
Second, the compensatory dose escalation often introduces gastrointestinal toxicity that patients struggle to tolerate.
Third, systematic monitoring of plasma levels remains scarce, leaving clinicians to guess at therapeutic sufficiency.
Fourth, the guidelines assume uniform patient metabolism, ignoring genetic polymorphisms that further modulate drug clearance.
Fifth, the financial implications of switching to expensive alternatives like darunavir are prohibitive in many health systems.
Sixth, the covert promotion of rifabutin as a “solution” is hampered by limited availability and regulatory hurdles.
Seventh, the interplay with NNRTIs such as efavirenz necessitates precise weight‑based dosing, a detail often omitted from primary care protocols.
Eighth, clinicians facing high patient loads may default to the simplest regimen, inadvertently compromising efficacy.
Ninth, patient education about these adjustments is essential, yet time constraints curtail thorough counseling.
Tenth, the cumulative effect of these oversights is an increased risk of drug resistance, undermining global HIV control efforts.
Ultimately, a more nuanced, patient‑centred approach is required to navigate these complex interactions.
Victoria Unikel
November 3, 2025 AT 14:26That’s a lot to take in, but it’s helpful.
AnGeL Zamorano Orozco
November 5, 2025 AT 08:06When I first read about rifampin’s enzymatic onslaught, it felt like watching a tragic play unfold on a microscopic stage.
The hero, our humble antiretroviral, strides onto the scene, only to be blindsided by the villainous inducer that roars “CYP3A4!” and shatters its resolve.
Every dose adjustment becomes a battlefield, with clinicians wielding charts like swords, trying desperately to restore balance.
The side‑effects surge like thunder, hitting the gut, the liver, the mind, leaving patients trembling.
And then there’s the ever‑looming specter of resistance, a dark cloud that threatens to eclipse any hope of cure.
Doctors scramble, swapping rifampin for rifabutin, only to encounter supply shortages that feel like a cruel twist of fate.
Pharmacies run low, paperwork piles up, and the patient waits, eyes glazed, wondering if anyone even hears their silent plea.
Meanwhile, the labs spit out numbers-ALT, AST, viral loads-each one a cryptic message demanding interpretation.
We, the caregivers, become translators of a language written in enzymes and metabolites, a role we never signed up for yet cannot abandon.
Even the simplest explanations become tangled in medical jargon, leaving families to navigate a maze of brochures and appointments.
Yet amidst the chaos, moments of triumph sparkle, like when a dose tweak finally stabilizes the load and the patient smiles.
Those fleeting victories remind us why we endure the endless night shifts and relentless paperwork.
They also whisper a promise: that perseverance can outshine the darkest drug‑interaction storms.
So we march on, armed with guidelines, hope, and the stubborn belief that science will eventually tame the beast.
In the end, the story is not just about rifampin and HIV; it’s about humanity’s relentless quest to heal itself against all odds.
Cynthia Petersen
November 7, 2025 AT 01:46Wow, your dramatics could give a soap opera a run for its money-good thing we have real solutions hidden beneath the theatrics!
John Petter
November 8, 2025 AT 19:26The clinical recommendations outlined herein reflect a synthesis of current evidence and pharmacologic rigor.
Ellie Hartman
November 10, 2025 AT 13:06Keep up the diligent work; your careful attention to dosing will make a tangible difference for patients.